



EU MDR / IVDR Consulting
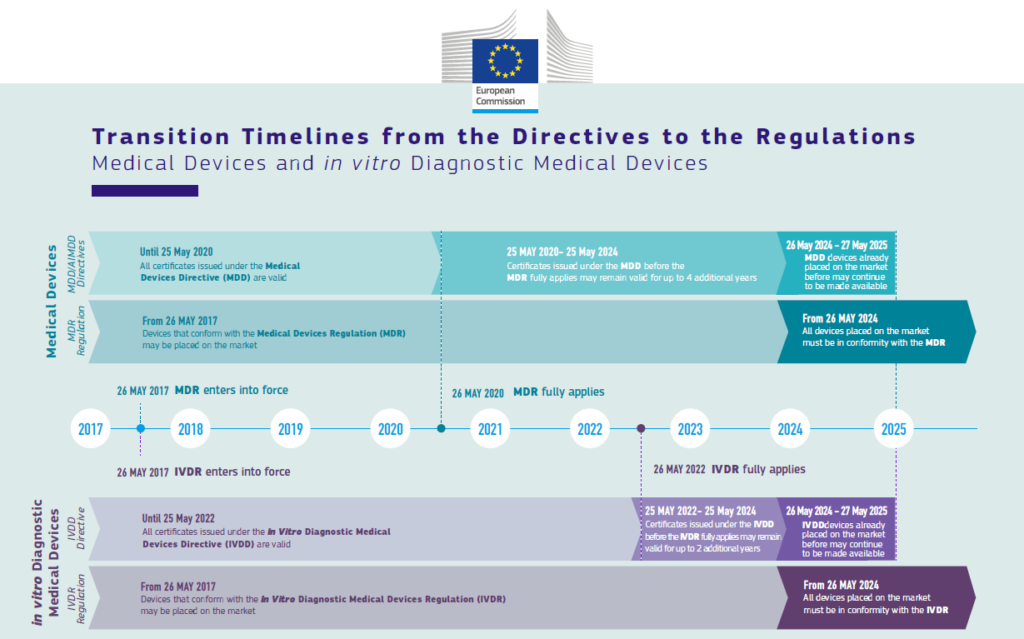
The new Medical Devices RegulationSearch for available translations of the preceding (EU) 2017/745 (MDR) and the In Vitro Diagnostic Medical Devices RegulationSearch for available translations of the preceding link (EU) 2017/746 (IVDR) bring EU legislation into line with technical advances, changes in medical science and progress in law-making.
The new regulations create a robust, transparent, and sustainable regulatory framework, recognised internationally, that improves clinical safety and creates fair market access for manufacturers and healthcare professionals. The European Commission intends to undertake a comprehensive evaluation of the MDR by May 2027.
Are you a medical device manufacturer looking to comply with the new European Union Medical Device Regulation (EU MDR) or In Vitro Diagnostic Regulation (IVDR)? Do you need expert guidance and support to navigate the complex regulatory requirements and ensure your products meet the highest safety and performance standards? Our team of experienced consultants can help.

At Techsol, we provide comprehensive EU MDR / IVDR consulting services to medical device companies of all sizes and specialties. Our team of regulatory experts, technical writers, and quality assurance professionals has a deep understanding of the new regulations and the unique challenges they pose for medical device manufacturers.
Why Choose Us?
- Ensure compliance with the new regulations and avoid costly delays and penalties.
- Enhance the safety and performance of your products, increasing customer confidence and market acceptance.
- Streamline preparation and submission of technical documentation, including the Technical File (TF) and Design Dossier (DD).
- Time complete the preparation of clinical evaluation reports (CERs) and provide guidance on the new clinical requirements under the EU MDR and IVDR.

How We Can Help
At Techsol, our expert team of Medical writers have an extensive research background, scientific knowledge, and ample experience in contributing to clinical development programs for global pharma companies.
Following are the key focus areas where our medical writing team has the expertise to deliver high-quality services throughout the drug / device development lifecycle as per applicable regulatory guidelines:
EU MDR/IVDR Support

> EU MDR Gap analysis of existing product dossier
> EU MDR/IVDR compliance strategy
> Changes in Notified Bodies
> Labelling review and updates
> Tech file remediation
> Design dossiers and renewals
> EUDAMED database management
> EU UDI implementation
> Regulatory strategy & Project deliverables US/EU/CA/ROW
> Marketing strategy support
EU-MDR Remediation Support
> Update clinical documentation from MED DEV Rev 4 to EU MDR
> Prepare robust Gap analysis at a detailed level for better Remediation execution
> Utilizing teams and maintaining confidentiality in Risk Management files, Restricted Materials documentation updates
> Verifying the need for PMCF and if needed, develop practical PMCF plans/designs
> Labeling remediation (IFUs, Content update, Artwork changes
> Remediation of Tech files (update or rewrite) & Publish
> Biocompatibility Assessments & Write BERs
IVDR Implementation Steps
|
STEPS |
TOOLS |
DELIVERABLES |
|
1. Scope and Plan |
Interviews, benchmarks, checklists |
Transition Planning, defining scope, timelines, methods, resources, structures, and tools including presentation to stakeholders |
|
2. Gap Assessment |
Impact assessment checklist for product, clinical and QMS |
Master Impact Matrix |
|
3. Portfolio Rationalization |
Product Portfolio Analysis template and remediation/retire cost budget template |
Report Product Portfolio Analysis including budget impact |
|
4. Global Impact Analysis |
Global Impact Analysis template |
Global impact report country by country |
|
5. Master Compliance Roadmap |
• Master Implementation Plan template • Project charter definition sheet • Resource requirements template • Steering committee report |
• Master Implementation roadmap • Governance model including project charters |
|
6. Regulatory Training |
• Specific in-house workshops • Detailed workshops MDR transition formats |
Alignment on changed SOP’s, and MDR compliance |
|
7. Implementation of Roadmap |
• Project charter progress sheet • Project charter status dashboard |
Implementation of the different charter projects |
|
8. Effectiveness Check |
Audits |
Resolve any discrepancies |
|
9. EU-MDR Compliance |
Notified Body Checklist |
Continued Compliance |



